At Syngoi, we work together with our customers to optimize their final product with our DNA. Thus, maximizing the functional response of the corresponding Gene of Interest to make advanced therapies safer and more effective.

OPTIMIZED TO PERFORM
A.
We have developed a cell-free scalable cGMP process to manufacture a project-based customized DNA product.
Our unique product is the combination of the corresponding Gene of Interest and proprietary adaptors which modules its properties according to project requirements and client´s final goal.
B.
Adaptors are key and a unique differential value of Syngoi’s Technology.
They are the close-ends of double stranded linear DNA, to optimize the characteristics of DNA, that is tailored for each project and client.
Are you considering to start a new project soon?
Are you considering to start a new project soon?
Are you considering to start a new project soon?
Are you considering to start a new project soon?
Are you considering to start a new project soon?
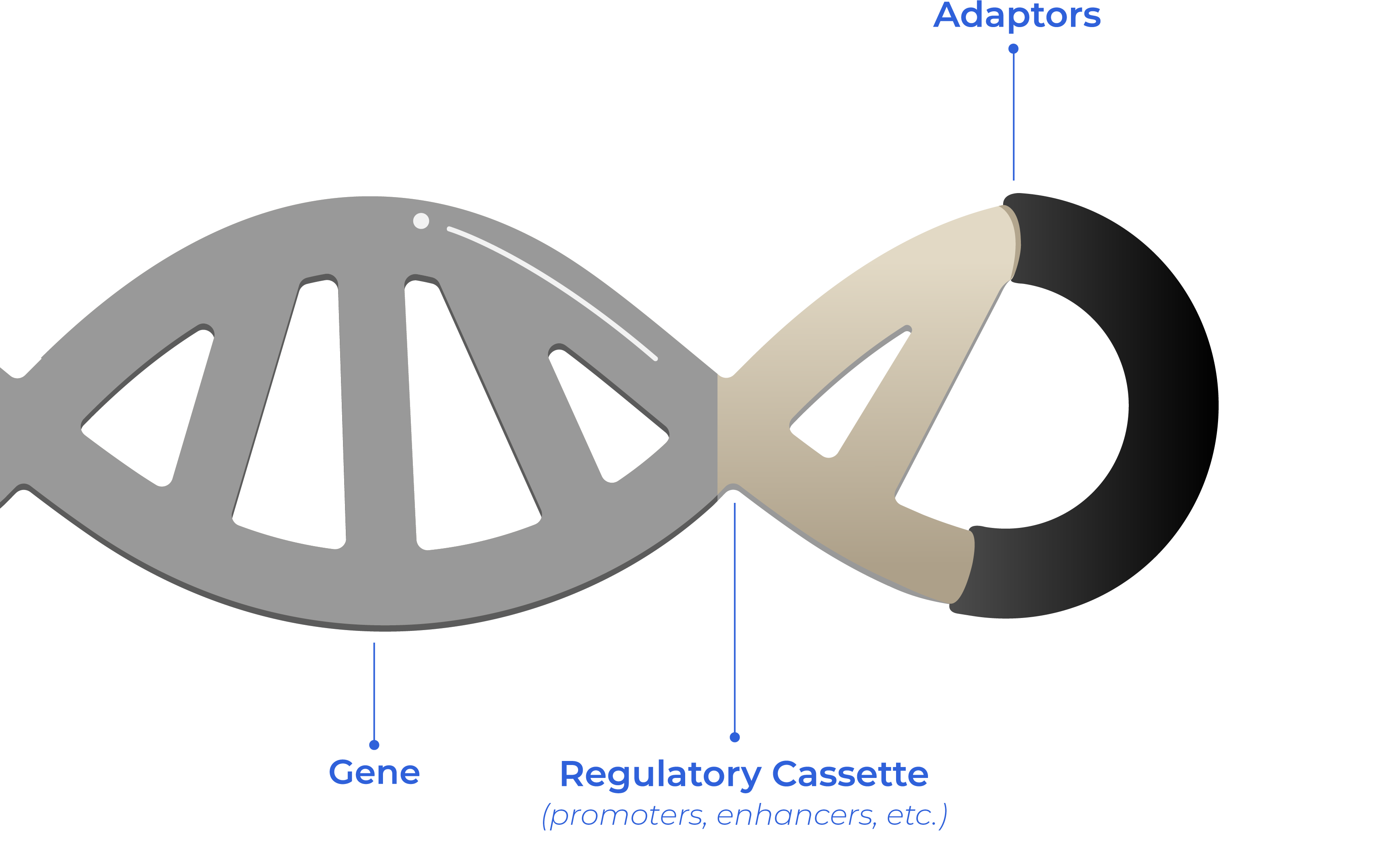
Optimized DNA is a unique synthetic double stranded linear close DNA made of the gene of interest which is close-ended by proprietary non-coding sequences called «adaptors» −project-based customized adaptors.
Library of ADAPTORS based on:
Natural nucleotides
Focused APTAMERS
NON-NATURAL nucleotides
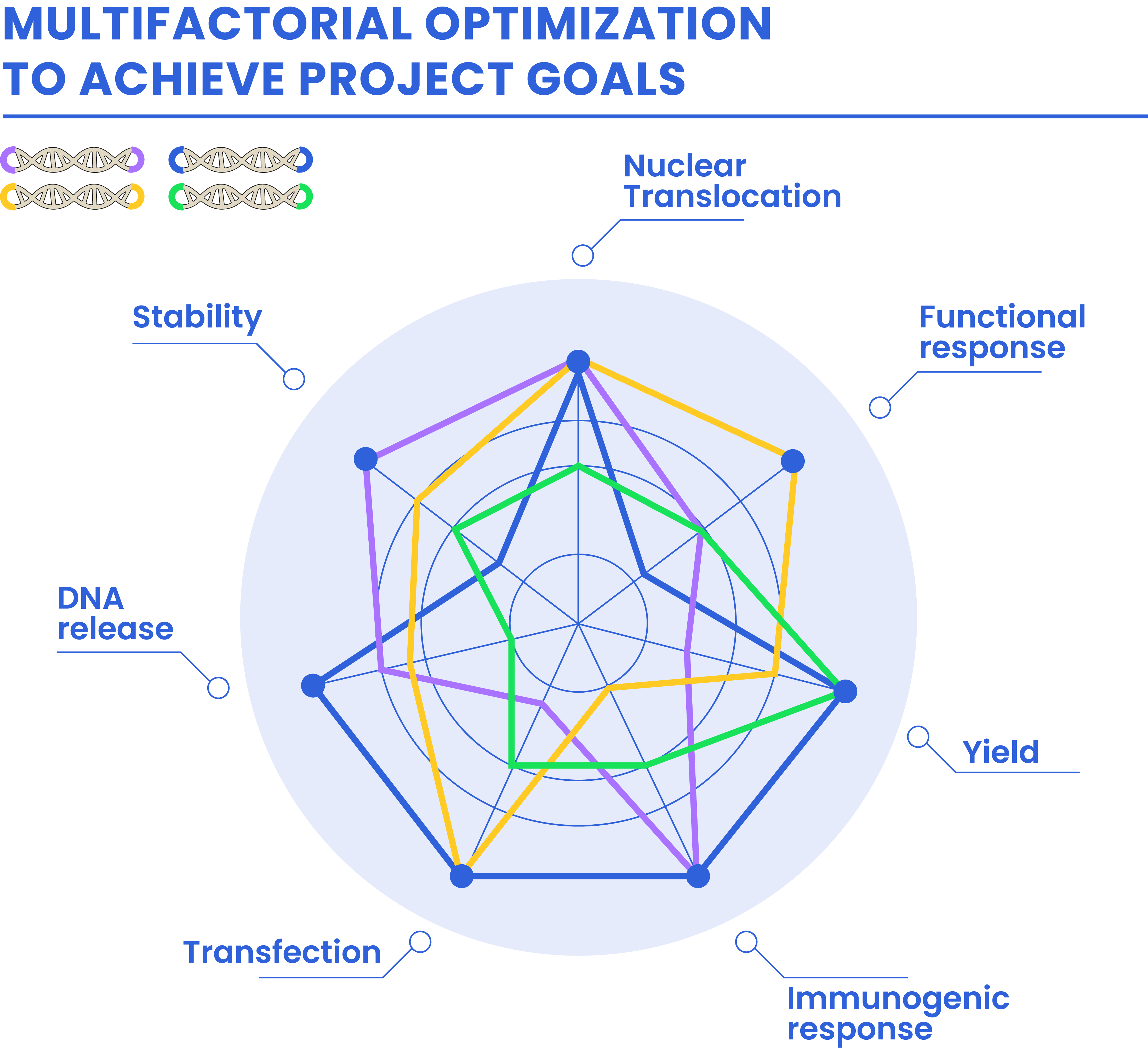
Key properties enhancement:
Stability
Immunogenic response
Release from delivery vectors
Nuclear translocation
OPTIMIZED DNA TO ACHIEVE CLIENTS NEEDS
Optimized DNA is the result of a multifactorial optimization by delivering customized adaptors that will match customer needs.
01. Linear Closed


02. Linear Open (double stranded)

03. Linear Open (single stranded)

NEW DIMENSIONS FOR ADVANCED THERAPIES, ONLY NEW PRODUCTS WILL DELIVER NEW RESULTS
We can combine a variety of adaptors to customized DNA according to client needs. In fact, many advanced therapies can benefit from our proprietary technology; from therapeutic applications: viral gene therapy, gene editing, CAR-T, non-viral gene therapy, vaccines,… to manufacturing solutions: AAVs, Lentivirus, mRNA…
NEW DIMENSIONS FOR ADVANCED THERAPIES, ONLY NEW PRODUCTS WILL DELIVER NEW RESULTS
We can combine a variety of adaptors to tailor customized DNA according to client needs. In fact, many advanced therapies can benefit from our proprietary technology; from therapeutic applications: viral gene therapy, gene editing, CAR-T, non-viral gene therapy, vaccines,… to manufacturing solutions: AAVs, Lentivirus, mRNA…
01. Linear Closed


02. Linear Open (double stranded)

03. Linear Open (single stranded)

OUR PROPRIETARY TECHNOLOGY ENABLES SOLUTIONS TO FULFILL THE NEEDS IN ADVANCED THERAPIES

Viral Gene Therapy
Non-Viral Gene Therapy
Genome Editing
DNA Vaccines

mRNA
01.
cGMP. Truly cGMP production, audited by regulatory agency.
02.
Multifactorial Optimization. Library of proprietary adaptors leads to module DNA properties and optimize them according to project requirements.
03.
Safety. No bacterial or antibiotic resistant DNA sequences.
04.
Scalability. Enzymatic process in a cell-free environment, small single-use bags; linear scale-up from miligrams to multi-grams.
05.
Delivery. cGMP manufacture in weeks instead of months.
06.
Gene of Interest. Encoding from short to long sequences, no limitation in terms of size, sequences type.



